Research Ethics Compliance Committee
The Research Ethics Compliance Committee (hereinafter referred to as the Committee or RECC), established at the IIRPS VU in 2024, assesses the compliance of planned research with research ethics requirements, assists researchers in the planning of their research in accordance with ethical principles, and provides advice and methodological support.
In assessing the compliance of planned research with research ethics, the Committee aims to protect the rights, dignity and well-being of human research subjects, to ensure compliance with research ethics, to assist researchers in the proper handling of data, and to minimise the risk of harm to research participants and the researchers themselves.
The Committee does not assess the quality of the planned research, does not monitor, evaluate or take responsibility for the research implementation process, does not carry out a data protection assessment in relation to the relevant legislation and, when a Data Protection Impact Assessment is needed (see “Frequently Asked Questions” below for more information), does not supervise its implementation.
Where planned research involves the participation of human subjects and/or their personal data (including but not limited to such data as social media posts), it is recommended to seek a review of compliance with research ethics from the Committee. If you decide to submit your planned research to the Committee for assessment, you can only do so before the research begins (exceptions are provided in paragraph 22 of the Regulations). If you plan to conduct a funded research project, the Committee should normally be contacted after funding has been received, at the beginning of the project, before the research itself begins.
Although it is not compulsory to apply to the Committee for the research you plan to carry out, you may be required to provide proof of approval from the Research Ethics Committee when you are involved in international research projects or when you wish to publish the results of your research.
You can apply to the Committee in Lithuanian or English. The Committee provides its services free of charge.
The work of the Committee is laid out in the Regulations approved by the Council of IIRPS VU.
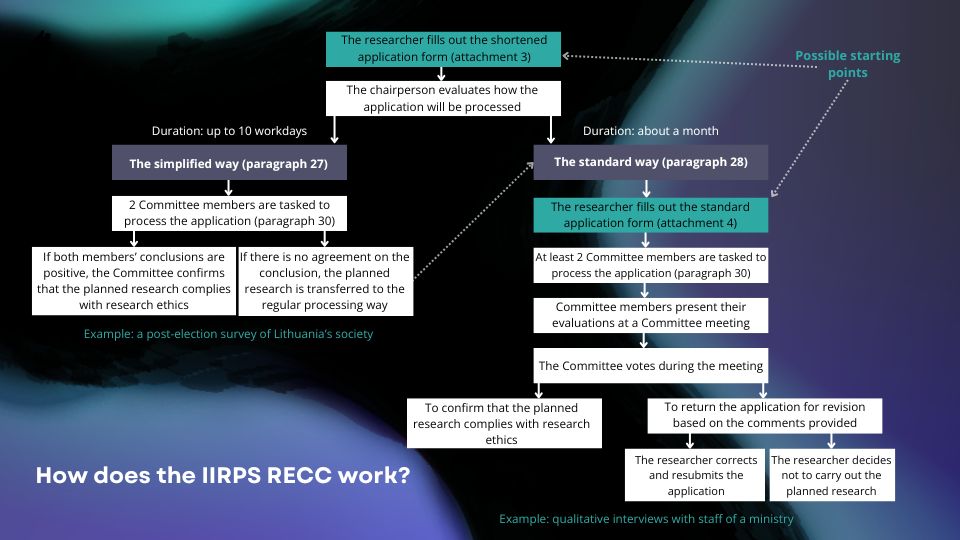
The procedure for assessing an application for compliance with research ethics for a planned study (see Chapter IV of the Regulations for more details):
- If you wish your planned study to be evaluated by the Committee, please fill in this shortened online application form.
- Once the Committee has received your shortened application, it will assess which way it should be examined: simplified or standard. The simplified way is quicker than the standard way, but it is only applicable to studies that are not ethically risky (see paragraph 25 of the Regulations for more precise criteria). If you are sure that your planned research does not meet the requirements for the simplified way, you can skip step 1 (submitting the shortened application) and instead email the Committee (amtek@tspmi.vu.lt) a filled-out standard application (see paragraph 4).
- If, after receiving your shortened application, the Committee decides that your planned study meets the requirements for simplified assessment, you will not be required to submit any additional documents. In this case, two members of the Committee will be appointed to examine your application in accordance with paragraph 30 of the Regulations. If both members give positive conclusions on the compliance of the planned study with research ethics, the Committee will vote on the approval of the planned study. If the Committee approves the compliance of the planned study with research ethics, you will be provided with a document in both English and Lithuanian proving this.
- If the Committee, after receiving the shortened application, decides that your planned research should be examined in the standard procedure or, if the planned research examined in the simplified procedure is not approved, you will be referred to the standard process (i.e. you will be asked to fill in a more detailed standard application form). In the “Committee meeting schedule” section below, you can find the latest date when you must submit a standard application for it to be examined and voted on at the next meeting. Upon receipt of a standard application, the Committee will appoint two or more members to examine the application. In accordance with paragraph 30 of the Regulations, the designated members will examine the application and present their findings at the Committee meeting. The Committee will then vote on whether to declare the proposed study to be in accordance with the principles of research ethics. If the Committee approves your planned study, you will receive a document in both English and Lithuanian. If the Committee approves your planned study without asking you to amend your application, the whole process of the standard evaluation way should take no more than one month. However, practice shows that the application usually has to be returned to the researcher 1-2 times for corrections. The actual processing time for a regular application is, therefore, somewhat longer and depends both on how quickly the researcher responds to the Committee’s comments and on the availability of the Committee members examining the application. The process is likely to take up to 2 months.
- If the Committee decides not to approve the planned study, you will be provided with comments on what needs to be corrected in the planned study in order to make it compliant with research ethics requirements. You will then have the option to revise the application (make changes to the application with the ‘Track Changes’ function) and resubmit it to the Committee for re-evaluation, or you will have the right to decide not to implement the study. A revised application might be processed more quickly than a completely new application (see paragraph 28.8 of the Regulations for more details).
The Committee members:
Dr. Ieva Petronytė-Urbonavičienė (the Committe Chair);
Dr. Konstantinas Andrijauskas;
The Secretary of the Committee:
Please find below the dates for the Committee meetings in the Spring semester of 2025. In the brackets next to each meeting date, you may find the deadline to submit your standard application for it to be voted on in that specific Committee meeting. If you submit your standard application later than the indicated submission deadline for a certain meeting, your application will be voted on in the next Committee meeting.
- 27 February (27 January)
- 27 March (26 February)
- 24 April (26 March)
- 29 May (23 April)
- 26 June (28 May)
When is it recommended to apply to the Committee for an evaluation of a planned study?
The Committee’s assessment can be requested when planning any research in the social sciences. However, it is recommended to seek it in cases when the planned research involves the participation of people and/or their personal data (including data such as social media posts or any other information that may be used to identify specific people).
In particular, the Committee should be consulted when planning to study vulnerable groups (minorities, minors, persons with disabilities, etc.), methodology requires not to disclose the true purpose of the research to the subjects, informed consent forms are not collected, high risks to the subjects are foreseen, unusual research methods are used, etc.
The least relevant cases for referral to the Committee are those where people are not being investigated (this includes not using social media posts, letters, and other documents created by people) or, if the research involves human research subjects, their personal data is not processed at any stage of the investigation, and where there are no additional risks referred to in paragraph 25.2 of the Regulations. However, even in such cases, the need to contact the Committee may be relevant if the researcher is aware that the project funder or the publisher of the publication may require approval from the Research Ethics Committee or if the researcher needs advice on the ethical aspects of the research. This type of research is most likely to be processed in a simplified way.
Why can’t I apply to the Committee for a study that has already been launched or is currently being implemented?
The primary purpose of research ethics committees, including the one at the IIRPS VU, is to protect human research subjects (and also researchers) from potential threats and harm to their rights, freedoms, dignity and well-being when they’re involved in research. The Committee’s task is, therefore, to assist the investigator in designing the study in such a way that these values are protected and the risks to people arising from the conduct of the study are minimised as much as possible. If the Committee’s assessment were to be carried out on a study that is already underway or is completed, the risks associated with the actions already taken by the researcher would no longer be manageable, and thus, this procedure would not fulfil the fundamental purpose of the Committee’s work.
There are a few exceptions where the Committee can be asked to evaluate research that has already been started (when a longitudinal study is planned or minor changes to the design of a study that the Committee has already approved are necessary), but even in these cases, the Committee may only evaluate the future phases of the study.
What should I do if a publisher requests ethics approval for a study that has been started or completed before the IIRPS VU Committee had been established?
Please contact Vilius Mačkinis, Deputy Director for Science and Research at the IIRPS VU (vilius.mackinis@tspmi.vu.lt).
Can students apply to the Committee?
IIRPS VU PhD students may apply to the Committee. The Committee does not consider planned research by BA and MA students. However, in exceptional cases, the Committee may process applications for research planned by BA or MA students if the supervisor of the student requests it and accepts the responsibility of submitting the application to the Committee. It is recommended that this exception be used only in cases where the supervisor has ethical concerns about the student’s research, such as researching vulnerable groups, controversial or socially sensitive topics, research involves high risks for the participants, etc., or where the student’s research is to be the basis of a publication.
Who is obliged to apply to the Committee for an ethics review of a planned study?
It is not compulsory to apply to the Committee. However, when planning a study, even if the researcher is able to manage all the ethical issues of the future study on his/her own, he/she should take into account that an increasing number of funders of research projects, and in particular publishers of publications (e. g. prestigious international journals), require a formal statement from the Research Ethics Committee that the study complies with the ethical standards. It should be noted that the Committee can only grant such approval for research that has not yet started.
What are the consequences for ignoring the Committee’s decision that the planned research is ethically inappropriate and carrying out the research regardless?
Although it is not compulsory to apply to the Committee, once you have applied to the Committee, you must follow the procedures set out in the Regulations. The Committee cannot simply prohibit you from carrying out your planned research. If the Committee decides not to approve the compliance of your planned study with research ethics, it will also comment on what needs to be corrected in order to make the planned study ethical. You will then be able to decide whether to amend your application and resubmit it to the Committee (in which case, your application may be subject to a slightly quicker examination process than a completely new application) or whether to decide not to carry out the study because the changes needed to make the study ethical are incompatible with the research goals. If a study is implemented without taking into account the comments made by the Committee and without its approval, the Committee will inform the investigator’s management and the Director of the IIRPS VU, informing them of the potential risks to the research participants and to the University’s reputation.
Who can contact the Committee?
The Committee is open to the IIRPS VU PhD students, staff and research groups where at least one researcher works at the IIRPS VU or is a PhD student of the IIRPS VU.
Can researchers from other Vilnius University faculties or other research institutions apply to the Committee?
Individual researchers who are not IIRPS VU staff members or PhD students are not eligible to apply to the Committee. However, if the research is planned to be carried out by a team where at least one investigator is an IIRPS VU staff member or PhD student, the team leader (or other designated team member) is entitled to apply to the Committee.
Are the services of the Committee chargeable?
The Committee’s services are free.
What languages can be used to apply to the Committee, communicate with the Committee, and in its approval?
The Committee’s communications may be in both Lithuanian and English. Submissions to the Committee can also be made in Lithuanian or English. The Committee’s conclusion on the compliance of the planned study with research ethics shall always be issued in both English and Lithuanian.
What is a Data Protection Impact Assessment? Does the Committee carry it out?
The Data Protection Impact Assessment (DPIA) is described in Article 35 of the General Data Protection Regulation of the European Union. A DPIA must be carried out where the planned processing of personal data (any information relating to an identifiable natural person) is likely to result in a serious risk to the rights and freedoms of individuals. A DPIA is a tool for describing a data processing operation and assessing the necessity and proportionality of such a processing operation, helping to manage the risks to the rights and freedoms of natural persons arising from the processing of personal data, including by assessing the risks and identifying measures to address those risks.
If you intend to process personal data in any of the following ways, your planned research will require a DPIA.
a) Without the consent of the subjects;
b) Personal data of people of vulnerable groups;
c) Personal identification codes;
d) Data from a population larger than 1% of the entire Lithuanian population;
e) Biometric or genetic data;
f) Video surveillance recordings in premises or areas not under the control of Vilnius University;
g) Audio-visual or audio recordings of conversations (by telephone or similar);
h) Data processing using technologies not previously used by the researcher;
i) Assessment of the personal aspects of minors (ethnic origin, academic performance, health, attitudes, etc.);
j) Video surveillance of employees for control purposes;
k) Automated decision-making (by technological means, without any human intervention);
l) Subjects’ location data;
m) Private correspondence, letters, emails, social networks, etc;
n) Transfers to third countries.
If the Committee, upon receipt of your application and taking into account the criteria listed above, and the DPIA previously carried out by the IIRPS VU does not cover your planned research, you will be informed and referred to a consultation with the VU Data Protection Officer (dap@vu.lt) who will assist you in carrying out the DPIA. The Committee does not provide advice on DPIAs. If the need for a DPIA is identified, this will not mean that your planned study will not be given a positive conclusion on compliance with research ethics on its own, but you will be required to carry out a DPIA because this is required by law.
Following a public call for applications, all IIRPS VU staff members and PhD students may nominate candidates to the Director of the IIRPS VU for membership in the Committee. Nominations may be made by a member of the staff or a PhD student of IIRPS VU. The Director shall then present the nominations to the IIRPS VU Council for a vote. The composition of the Committee, consisting of at least five members (including the Chairperson), shall be approved for 3 years. The Committee shall be composed considering the candidates’ professional qualifications, competences, experience in research, research ethics, data protection and management, and the maintenance of a gender and researcher career stage balance among the members.
All members of the Committee shall sign a confidentiality pledge and a declaration of impartiality before beginning their work on the Committee and, in the event of a conflict of interest in the assessment of certain applications, shall be required to recuse themselves from examining those applications. In the performance of their duties, the members of the Committee shall be guided by the principles of respect for human rights and dignity, transparency and other principles set out in the Code of Academic Ethics of Vilnius University, the codes of professional ethics, other documents governing research ethics, and these Regulations.
The Director of the IIRPS VU shall also appoint a Secretary of the Committee, who shall not have the rights of a member of the Committee but shall assist the Committee with administrative work.
Members and the Secretary shall be reimbursed in accordance with the procedures laid down by the VU.
Does the Committee assess the quality of the planned studies?
The Committee’s competency is limited to assessing the planned study’s details related to research ethics issues. Methodological choices, aims, objectives, and other aspects of the study that are not ethically relevant are not evaluated by the Committee.
Does the Committee oversee the implementation of the study for which it has granted approval?
The Committee shall not oversee the implementation of the study for which it has granted approval, including the ethical aspects of the research. This is the responsibility of the investigators themselves.
What should I do if a study approved by the Committee requires a change in study design? Is the Committee’s conclusion still valid?
For the Committee’s conclusion to remain valid, changes to the study design must be approved by the Committee.
If, in the course of a study already approved by the Committee, minor changes to the study design become necessary, a revised application has to be submitted to the Committee in accordance with the procedure provided for in paragraphs 28.7 and 28.8 of the Regulations. The process of evaluating non-substantive changes would take less time than the full standard process.
If a significant change in research design already approved by the Committee and being implemented is required, such research is treated as a new planned study and must go through the standard evaluation process when an updated standard application is submitted.
If you submit a revised application for a study that has already been approved by the Committee and has already started to be implemented, the Committee will decide whether the changes should be considered minor or major. Pending the Committee’s new conclusion, you may not proceed with the aspects of the study related to the amendments.
What should I do if the implementation of a study approved by the Committee has been delayed beyond the validity term of the Committee’s conclusion?
The Committee’s decision that the planned research is in accordance with research ethics is valid for a limited period of time, to be decided on a case-by-case basis according to the duration of the planned research, plus an additional period of time after the planned research is expected to end. This time limit may be extended at the request of the investigator by submitting a reasoned request to the Committee in this form, provided that the continued or planned research would be carried out in exactly the same way as indicated in the previous application approved by the Committee. The decision to extend the validity of the Committee’s decision shall be taken by the Chairperson of the Committee.
What are the most common aspects to be corrected in applications?
The terms ‘anonymisation’ and ‘pseudonymisation’ are confused or used interchangeably. Anonymisation of data (e.g. interview transcripts) means removing any details that could directly or indirectly identify a person (not only name or contact details but also specific job titles, special characteristics of a person, etc.). Anonymisation, when properly carried out, makes data very safe to process and publicise, but this process is time-consuming, requires a high degree of diligence, is difficult to carry out flawlessly, and, in some cases, may mean destroying parts of valuable data to ensure that the person concerned is no longer possible to identify. Pseudonymisation is a simpler and more commonly used method. Pseudonymisation involves giving an alias or a code to a research participant and keeping the file connecting the real name and the code or alias separate from the rest of the data and only accessible to the researcher. Other information in the data that could potentially lead to indirect identification shall not be destroyed. It should be noted, however, that the pseudonymisation method still usually avoids leaving details that could easily lead to indirect identification in quotations used in publications.
The application indicates that an artificial intelligence (AI)-based tool will be used to process the data (transcription, analysis, etc.), but does not specify the tool and how it would ensure the security of personal or other sensitive data. Please identify the specific AI tools that would be used and justify why they are considered secure in terms of personal and other sensitive data when submitting the application.
Inconsistencies regarding the data retention periods and other aspects of the research in the application, the information sheets and the informed consent forms attached to the application. Please make sure that there are no discrepancies in the application and its annexes.
It is stated that participants may withdraw from the study and withdraw their data up to the time of the release of publications based on that data. While this flexibility towards study participants is welcome, it should be borne in mind that this may be difficult or impossible to implement in practice. It is advisable to set an adequate period, e.g. several months, between the last opportunity for participants to withdraw from the study and the release of the publications. Then, the withdrawal of a research participant will allow sufficient time for the publications to be adjusted accordingly before their release.
- Ethics in Social Science and Humanities (The European Commission)
- Guidelines for assessing compliance with research ethics (Ombudsman for Academic Ethics and Procedures)
- Regulations of Faculty Ethics Assessment Committee (Humanities, Utrecht University)
- Regulations of the Joint Committee on Compliance with Research Ethics (Vilnius University, Faculty of Philosophy)
- More useful resources on research ethics (European Network of Research Ethics Committees)
- Information on data protection impact assessment and personal data register (Data Protection Officer, Vilnius University).
The Committee’s email address: amtek@tspmi.vu.lt (submission of applications and consultations).
The Secretary of the Committee: Paulius Vijeikis (paulius.vijeikis@tspmi.vu.lt) (administrative inquiries).
Viktoras Bulavas, Data Protection Officer at Vilnius University: tel. 2366202, email: dap@vu.lt (data protection issues, data protection impact assessment and personal data processing).







